2025 Update: A Year of Growth, Collaboration, and Real Progress
2025 was a major growth year for CureMFM13 - shifting from planning to real progress in research, awareness, and community building. We secured formal disease classification (OMIM #621078) and an official name: Myofibrillar Myopathy type 13 (MFM13) with rimmed vacuoles, which boosted recognition and ended confusion from multiple labels. We rebranded from CureHSPB8, expanded our outreach and resources, and grew our community - adding 8 newly identified patients and increasing newsletter and social engagement. On the research side, we advanced patient-derived models, launched new collaborations (including the first partially humanized mouse model), and began developing mutation-specific tools to accelerate future therapies.

2025 Update: A Year of Growth, Collaboration, and Real Progress
The year 2025 was an important year of growth CureMFM13. Building on the work started in 2024, we moved from planning to real progress in research, awareness, and community building. It was a year of expansion - of our team, our research efforts, and our visibility within the myopathy field
One of the major achievements of 2025 was the formal disease classification. Securing an OMIM number (#621078)and an official disease name for conditions caused by HSPB8 frameshift mutations significantly increased recognition and visibility. The disease is now formally classified as Myofibrillar Myopathy type 13 (MFM13) with rimmed vacuoles.
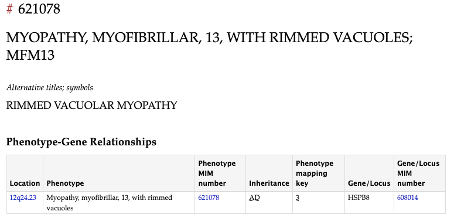
MFM13 is an ultra-rare disorder caused by eight different mutations resulting in two distinct toxic gain-of-function frameshift options (Fig. 2). The use of multiple names for the same disease greatly complicated research efforts and delayed diagnosis. Thanks to our advocacy and coordination efforts, the disease now has an official name, representing a critical milestone for both the scientific and patient communities.
This change also motivated a comprehensive rebranding process. Many of you may remember us as CureHSPB8, but to align with the new disease classification and improve clarity, we decided to rebrand and update our identity and core communication materials [curemfm13.org].

Among many parallel activities, we placed strong emphasis on increasing the visibility of MFM13 and raising disease awareness. Throughout 2025, we participated in several scientific and patient-focused conferences, strengthening our presence within the rare disease and myopathy communities.
Our Founder, Todd King, and Director, Anna Kordala, were interviewed by Jessica Lynn for RAREatives in the feature “CureMFM13: How a Rare Disease Diagnosis Built a Community.” In this interview, they shared the story of CureMFM13 and how a rare disease diagnosis became the foundation for building a connected and empowered community.
Our communication channels also increased significantly. Our newsletter community grew from 40 to 253 subscribers - people who actively want to follow our progress and stay informed about our work. We are deeply grateful for this growing engagement and support. At the same time, our social media presence in LinkedIn increased from approximately 60 to 354 followers, there and in other platforms, we regularly share updates about the CureMFM13, research progress, and newly published scientific studies in the MFM13 field.

To support researchers, clinicians, and interested families, we expanded our website. We created a dedicated librarythat brings together key publications related to the molecular basis of MFM13, the CASA complex, autophagy-related mechanisms, and therapeutic approaches. These resources are curated to make complex scientific information more accessible and centralized.
For those who prefer audio content, we launched a podcast focused on MFM13-related topics. In these episodes, we discuss disease mechanisms, summarize recent publications, and highlight case study reports, creating another accessible format for education and awareness.

All of these awareness and advocacy efforts contributed directly to expanding the known MFM13 patient community. In 2025, we found eight new individuals affected by MFM13, who now have access to shared knowledge, resources, and data. Recognizing that many families struggle for years with unexplained muscle weakness, we also updated our website with practical information on genetic testing. This includes an overview of available myopathy gene panels, single-gene testing options, and whole-exome and whole-genome sequencing possibilities, helping patients and clinicians navigate the diagnostic process more effectively.
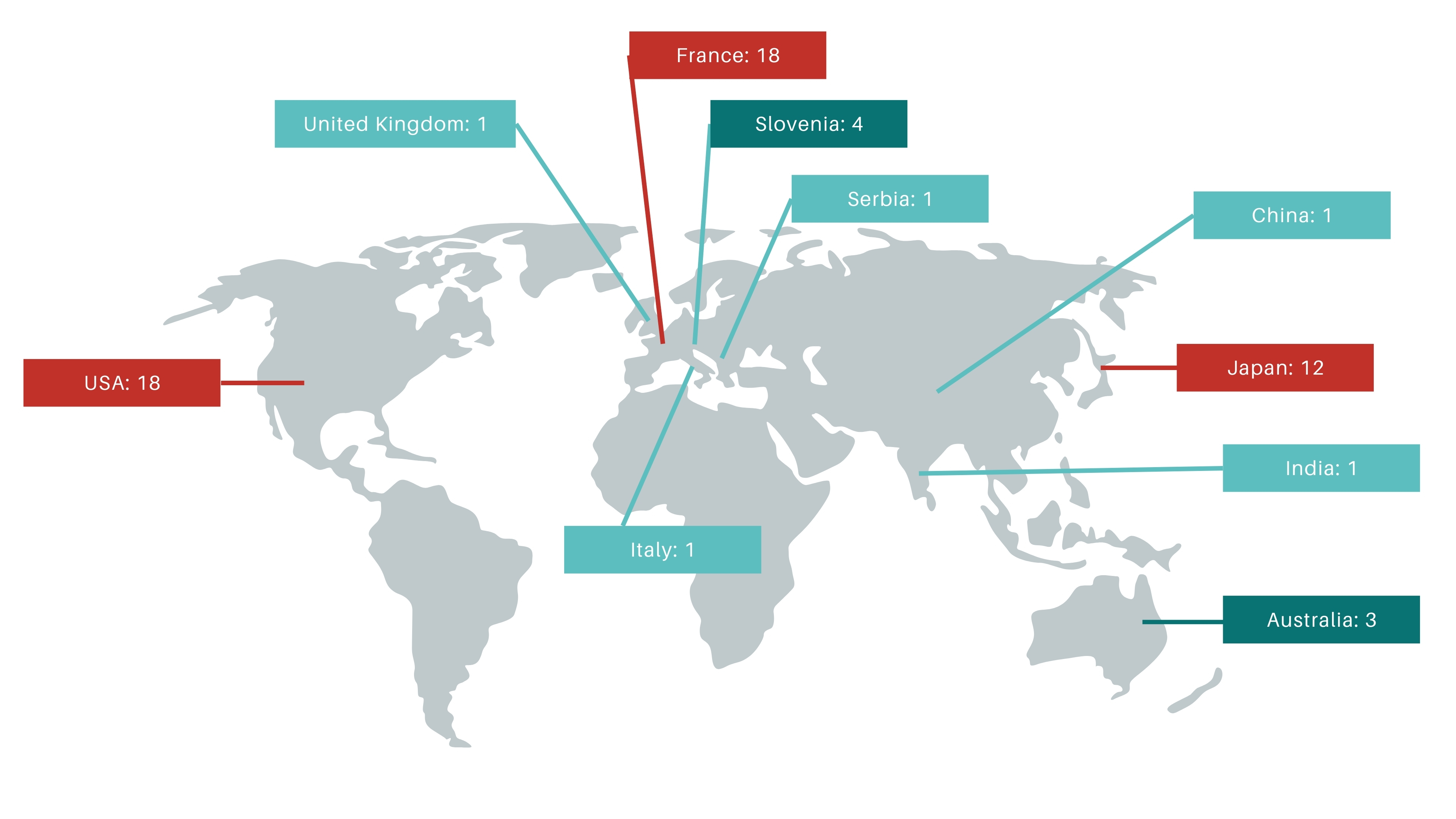
Overall, 2025 marked a transition from establishing visibility to building substance. While challenges remain, this year demonstrated what is possible through focused collaboration and strategic investment in research tools. We enter the next phase with stronger models, clearer priorities, and a growing, engaged community—bringing us closer to meaningful advances for people affected by MFM13.
Awareness and advocacy are not the only pillars of our work. In 2025, we placed a strong and deliberate focus on research and development, with the goal of expanding the research toolkit available to scientists and encouraging deeper investigation into the molecular mechanisms underlying MFM13.
Several key research resources are already available, but we are not stopping there. Patient-derived fibroblast cell lines and DNA samples can be accessed through the Coriell Institute, providing an important starting point for independent research efforts. We are actively developing induced pluripotent stem cells (iPSCs) derived from MFM13 patients carrying HSPB8 frameshift mutations, along with matched isogenic control lines. Following molecular validation, we are excited to make these models available to the research community.

We are particularly proud to have initiated a collaboration with the International Institute of Molecular and Cell Biology (IIMCB) in Warsaw to generate the first partially humanized MFM13 mouse model. This represents a major milestone for the field. We are eagerly awaiting initial results and we are already planning detailed phenotypic characterization studies scheduled for 2026.

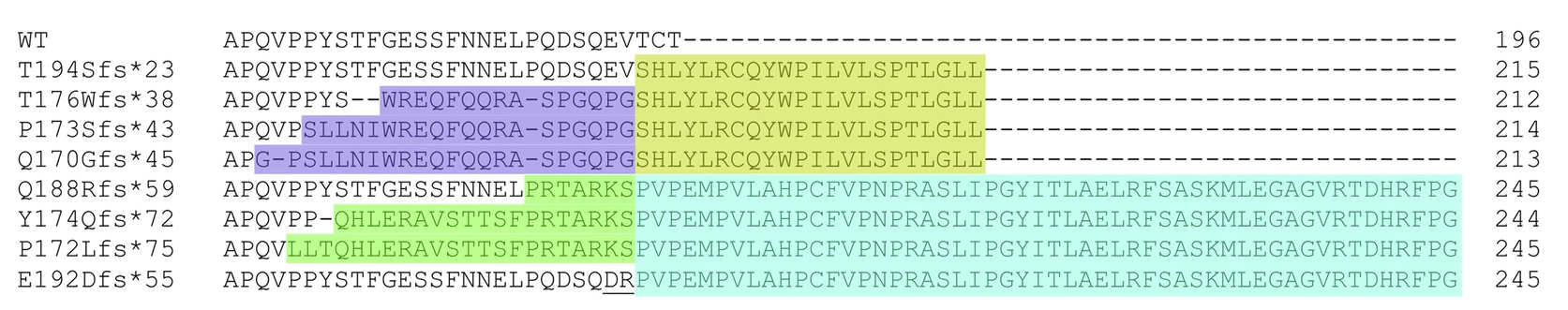
Because currently available HSPB8 antibodies do not distinguish between wild-type and mutant protein, we made a strategic decision to develop a mutation-specific HSPB8 antibody targeting the fs1 frameshift variant (for detailed mutation context, see Tedesco et al., 2023). This tool will be critical for studying disease-specific protein behavior and toxic gain-of-function mechanisms.
A mission of CureMFM13 is to improve the lives of all people affected by MFM13 and their families. We do this by accelerating the drug development process, building a strong and empowered community, and advocating for the community. For this reason, we are placing a strong emphasis on drug development, with a particular focus on drug repurposing strategies and novel therapeutic approaches. We actively welcome collaboration proposals and input from academic groups and companies with expertise in drug discovery, development, and translational research.
Our long-standing collaboration with the Professor Angelo Poletti laboratory at the University of Milan continues to thrive. Dr Barbara Tedesco’s work has made a significant impact on the MFM13 field, and her 2025 publication highlights the critical contributions of the Poletti team to understanding disease mechanisms. We are also pleased that Veronica Marchesi, whom we had the pleasure of meeting during her master’s studies in Milan, has now officially begun her PhD and is continuing her research on MFM13.

This year was full of important achievements in awareness, advocacy, and research development, but it also highlighted ongoing challenges faced by the MFM13 community. While we have made progress in visibility and knowledge sharing, significant diagnostic barriers remain. We are still advocating for HSPB8 to be included in more myopathy panels, as it is currently mostly available only in neuropathy panels, which affects timely and accurate diagnosis for MFM13 patients.
At the same time, we are continuing our efforts to identify reliable biomarkers and to direct our focus toward drug research.
Our main goals have not changed - we want a life free of MFM13 disease.
- Our mission is to improve the lives of all people affected by MFM13 and their families. We do this by accelerating the drug development process, building a strong and empowered community and advocating for the community.
- Our vision is life free of MFM13 and all its burden.
- Our purpose is to alleviate suffering and empower the patients
- Our strategy is to invest in R&D, support MFM13 families, advocate for the community and grow as an organization
- Our values are urgency, transparency, evidence-based
