Summary - Tedesco
A important study by Tedesco et al., published on May 2025, that is collaboration of Angelo Poletti and Viriginia Kimonis ' laboratory teams titled “C-terminal HSPB8 frameshift variants define a toxic gain-of-function signature in MFM13”, in the European Journal of Human Genetics, identifies three novel frameshift mutations in HSPB8 associated with Myofibrillar Myopathy 13 with Rimmed Vacuoles (MFM13). This study builds on existing knowledge and offers new insight into how C-terminal elongations of HSPB8 result in toxic protein aggregation, motor impairment, and multisystem involvement.

An important study by Tedesco et al., published on May 2025, that is collaboration of Angelo Poletti and Viriginia Kimonis ' laboratory teams titled “C-terminal HSPB8 frameshift variants define a toxic gain-of-function signature in MFM13”, in the European Journal of Human Genetics, identifies three novel frameshift mutations in HSPB8 associated with Myofibrillar Myopathy 13 with Rimmed Vacuoles (MFM13). This study builds on existing knowledge and offers new insight into how C-terminal elongations of HSPB8 result in toxic protein aggregation, motor impairment, and multisystem involvement.
🔍 What Are the New Mutations — and How Are They Unique?
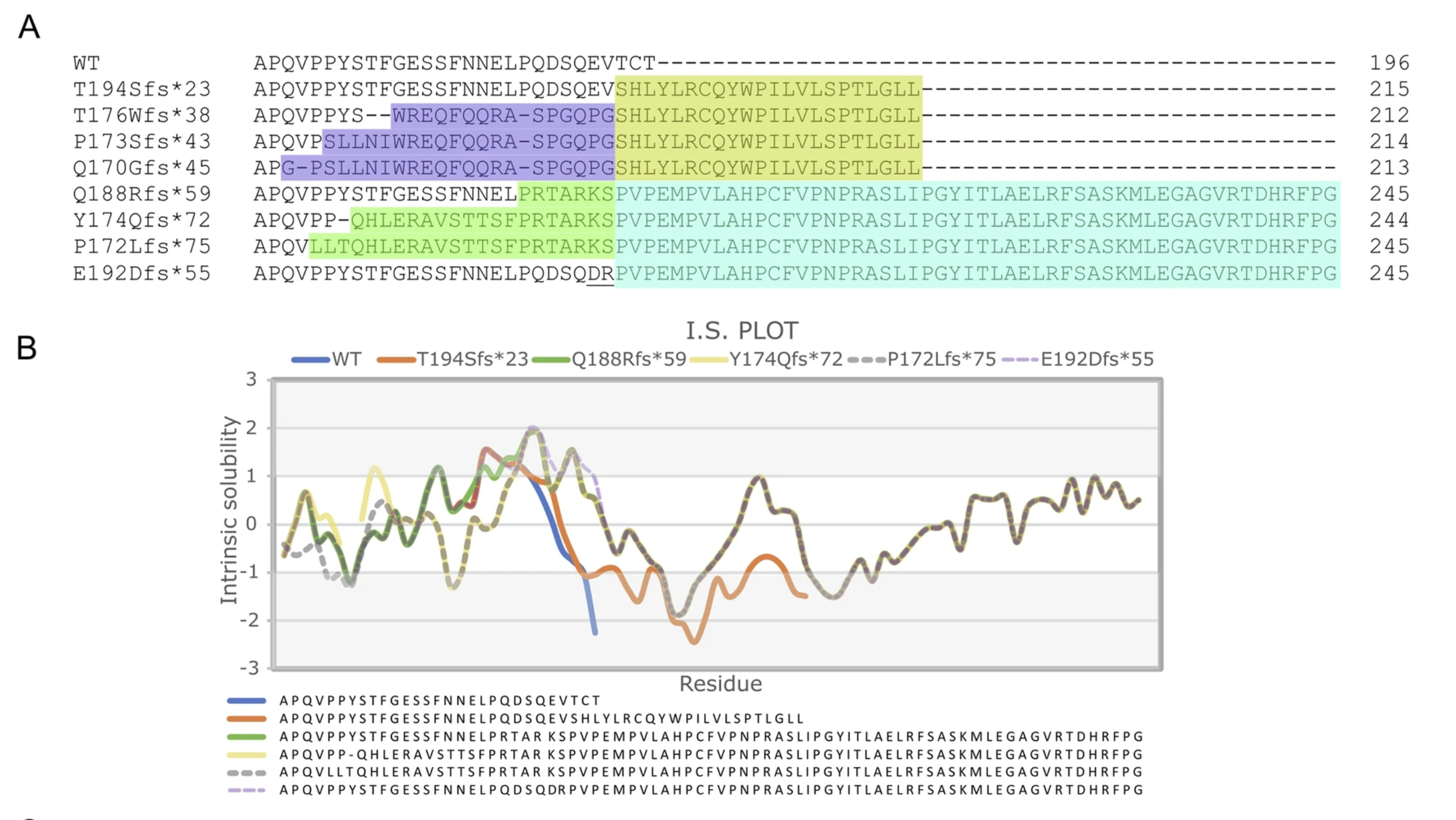
The newly identified mutations— p.Q188Rfs*59, p.Y174Qfs*72 and p.P172Lfs*75—all cause elongation of the HSPB8 C-terminal tail by adding aberrant amino acids beyond the normal stop codon. Although these variants arise from different genetic alterations, they all result in the same frameshift, producing an identical C-terminal sequence. However, depending on the mutation’s position within the gene, the frameshift may begin earlier (e.g., p.Y174Qfs*72) or later (e.g., p.Q188Rfs*59), leading to greater or smaller differences between the mutant and wild-type proteins (Fig. 1).
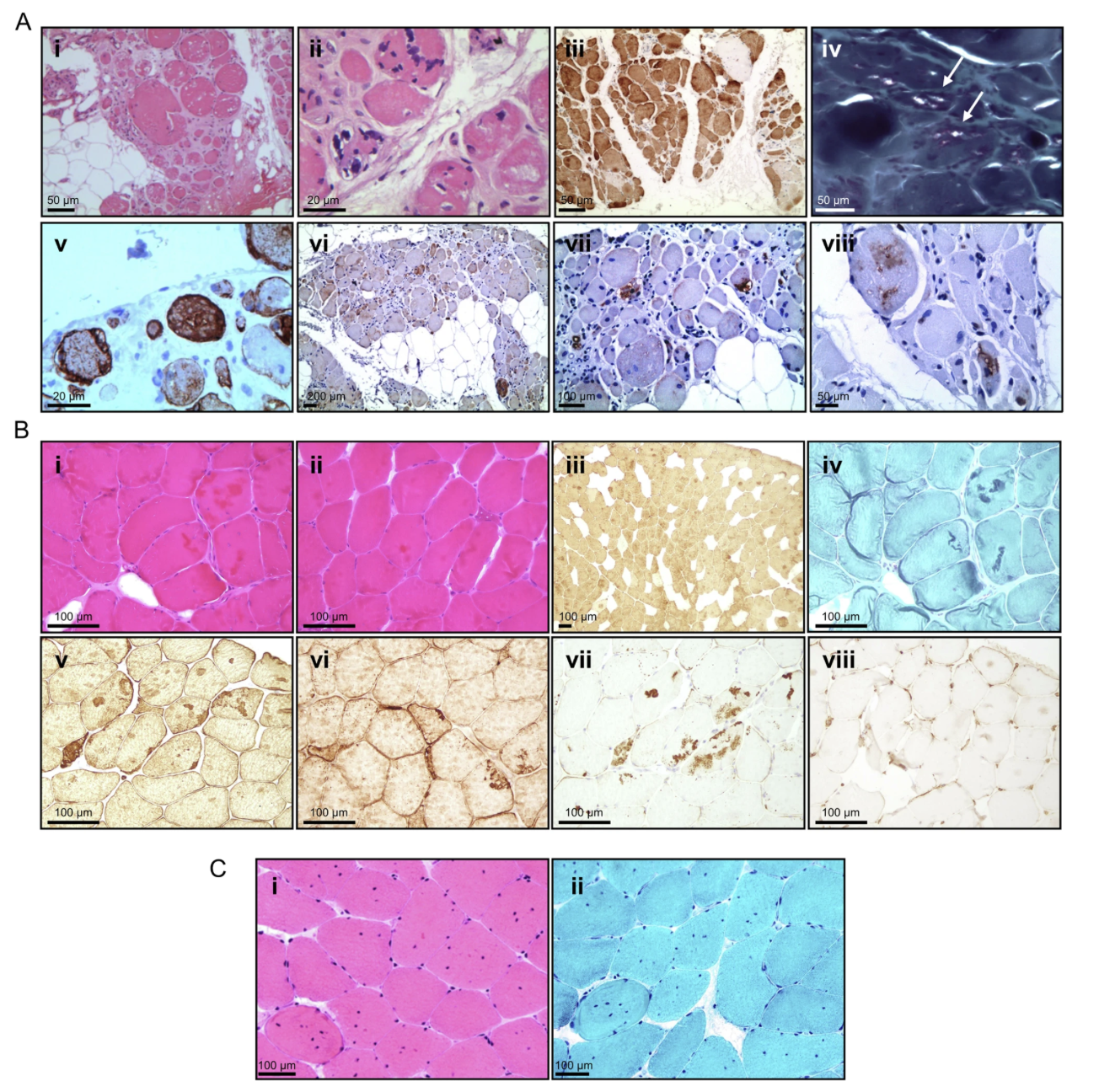
Clinically, individuals carrying these mutations exhibit progressive muscle weakness affecting both proximal and distal muscle groups. Notably, these cases also involve significant respiratory and cardiac complications, features that appear more prominent than in many previously reported MFM13 cases. This suggests a potentially broader and more severe disease spectrum. Muscle biopsies reveal classic signs of myofibrillar myopathy, including rimmed vacuoles and cytoplasmic protein aggregates, further supporting the pathogenicity of these newly defined variants (Fig. 2).
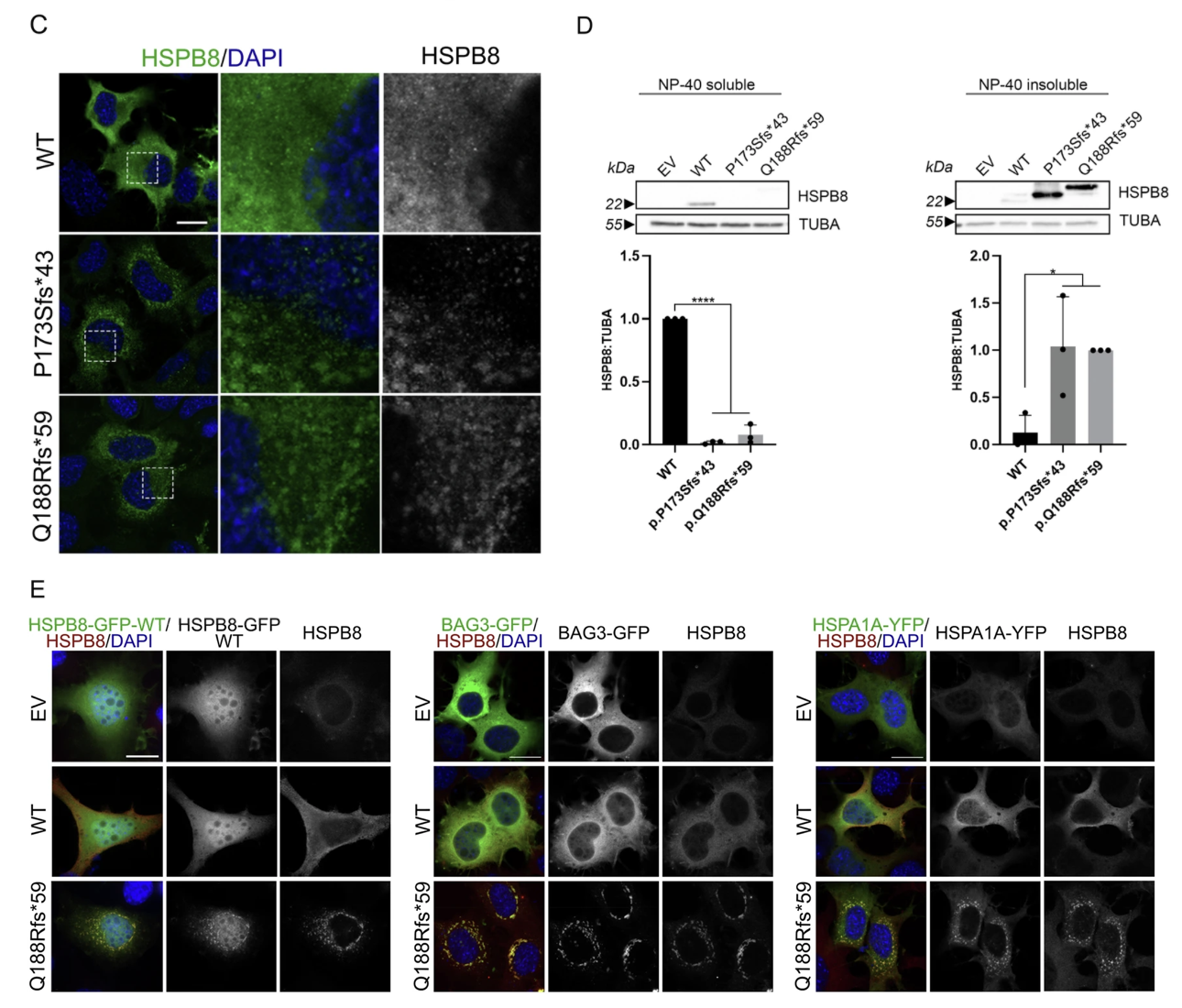
Using transient transfection of human cell lines, the researchers expressed these mutant HSPB8 proteins and observed the following:
- All three mutant proteins formed large cytoplasmic aggregates, which co-localized with known autophagy markers such as p62 and LC3B, indicating impaired protein clearance.
- The mutant HSPB8 sequestered CASA complex partners—BAG3 and HSP70—into aggregates, thereby disrupting their normal chaperone-assisted selective autophagy (CASA) function.
- Cells expressing the mutants showed signs of endoplasmic reticulum (ER) stress and activation of the unfolded protein response (UPR) pathways, reflecting broader cellular dysfunction.
- Even under conditions that typically enhance autophagy (e.g., treatment with autophagy-inducing agents), mutant HSPB8 aggregates persisted, indicating resistance to normal degradation mechanisms.
These findings strongly support a toxic gain-of-function mechanism, whereby the presence of the mutant protein—not merely its absence—actively disrupts cellular homeostasis.
Crucially, the study demonstrates that the newly defined elongated C-terminal tail itself is likely the key driver of toxicity.
How Do These Findings Build on What We Already Know?
Earlier work, including a 2023 study by Tedesco et al., had already provided experimental evidence that frameshift mutations in the C-terminal region of HSPB8 are pathogenic—primarily due to their tendency to form toxic aggregates and impair the function of the CASA complex, a critical system for maintaining protein homeostasis.
The new 2025 study builds on this foundation by identifying and characterizing newly diagnosed C-terminally elongated variants. Despite originating from different genetic mutations, these variants all lead to a similar, toxic protein behavior—marked by aggregation and disruption of autophagy pathways—highlighting a convergent pathogenic mechanism driven by the altered C-terminal tail.
Clinically, these new variants are associated not only with progressive muscle weakness but also with pronounced respiratory and cardiac involvement. This broader symptom profile suggests that these mutations may define a more severe and systemic form of MFM13 than earlier variants.
Why This Study Matters
- Confirms toxic gain-of-function model via in vitro evidence
- Links C-terminal elongation with convergent pathogenic behavior
- Expands known phenotypic spectrum, including systemic involvement
- Sets the stage for targeted therapies aimed at toxic C-terminal tails or protein clearance
The authors advocate for therapeutic approaches that reduce mutant HSPB8 levels or boost autophagy, echoing the strategy outlined in earlier reviews.
📖 Read the full article here: https://www.nature.com/articles/s41431-025-01868-z
Podcast: Episode 7
Social media: LinkedIn, X, Facebook, BlueSky, X.
